Abstract
Oxidative stress (OS) has been demonstrated to play a central role in the pathophysiology of sickle cell disease (SCD). The antioxidant N -acetylcysteine (NAC) is an important precursor of cysteine, which is the rate limiting substrate for glutathione (GSH) synthesis, and can thereby exert intracellular antioxidant effects. Yet, because of its thiol group, NAC can also act extracellularly as a reducing agent to break protein disulfide bonds, or to scavenge reactive oxidants. Pilot studies with NAC in SCD have demonstrated various beneficial effects, reducing markers of red blood cell membrane damage, hemolysis and OS. The major plasma modulator of OS is the amino acid cysteine (Cys), with the ratio of its reduced form to the oxidized form reflecting the plasma redox state. Under oxidative stress, relatively low levels of free thiol Cys and high levels of protein bound Cys are observed (Fu et al. Blood 2015; 126:1044). The aim of this study was to assess the effects of oral NAC on the thiol redox balance in SCD patients. Moreover, we explored correlations of the thiol redox balance at baseline with other clinical and pathophysiological markers of SCD.
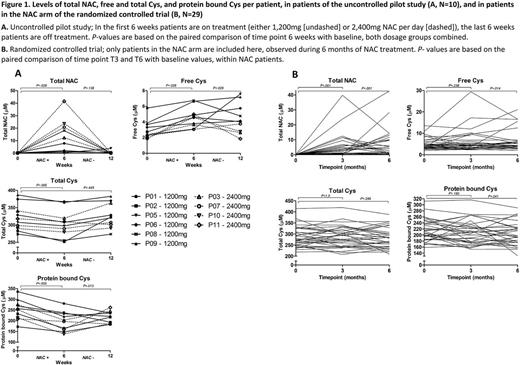
Plasma was analyzed from 2 completed prospective studies that evaluated the effect of oral NAC in SCD: 1) an open label, uncontrolled, pilot study consisting of 6 weeks of NAC 1,200 -2,400mg daily, and 6 weeks follow-up in patients with HbSS or HbSβ⁰-disease (N=10, Nur et al. Ann Hematol. 2012; 91:1097-105); and 2) a randomized, placebo-controlled trial (RCT) of oral NAC 1,200mg daily for 6 months in HbSS, HbSC, HbSβ⁰ or HbSβ⁺ patients (N=96, Sins et al. Br J Haematol. 2017 ) . Using liquid chromatography-tandem mass-spectrometry, we assessed the following markers of the thiol redox balance: total NAC, total and free (reduced) Cys, and protein bound (oxidized) Cys.
In both studies, oral NAC significantly increased levels of total NAC and free Cys, and decreased levels of protein bound Cys, as compared to baseline (figure 1). In the pilot study, total Cys was decreased as compared to baseline, however, no significant difference was observed in the RCT. In addition, when comparing the mean change from baseline of these parameters between the NAC arm and the placebo arm of the RCT, similar results were found. Yet, differences in mean change of protein bound and total Cys did not reach statistical significance in this analysis (results not shown).
Multivariate linear regression analysis of baseline thiol redox values in the RCT was performed, adjusting for genotype and use of HU. In this analysis, higher levels of free Cys (less OS) were independently associated with a higher MCV (standardized β [sβ] 0.36, P<.001), TAT (sβ 0.19, P=0.040) and levels of Nε-(carboxymethyl)lysine (CML, advanced glycation end product; sβ 0.27, P=.005), lower sP-selectin levels (sβ -0.33, P=.001) and a lower number of hospital admissions in the 3 years prior to randomization (sβ -0.21, P=.027). Higher levels of protein bound Cys (more OS) were independently associated with VWF:ag levels (sβ 0.24, P=.016) and the HbSS/HbSβ0 genotype (sβ -0.25, P=.020). Lastly, higher levels of total Cys (more OS) were independently associated with older age (sβ 0.21, P=.027) and the HbSS/HbSβ0 genotype (sβ -0.21, P=.042), and higher levels of VWF:ag (sβ 0.28, P=.004), CML (sβ 0.17, P=.046) and prothrombin activation fragment F1-2 (sβ 0.18, P=.038).
In line with previous intravenous studies, oral treatment with NAC significantly improved the thiol plasma redox balance in patients with SCD. These effects appeared to be most apparent in the uncontrolled pilot study, possibly due to poor adherence and a relatively low dosage in the RCT. Nevertheless, these findings emphasize the role of NAC as an extracellular reducing agent of protein disulfide bonds, beyond acting as a substrate for glutathione synthesis or as a direct scavenger of reactive oxygen species. Moreover, a higher extracellular oxidant burden at baseline was independently associated with endothelial and coagulation activation, and with the number of hospital admissions. Thereby, we provide indirect evidence that improvement of the thiol redox balance may be an important therapeutic target in SCD, improving other pathophysiological processes of the disease, and potentially even reducing the number of hospital admissions. Hence, the use of NAC and other thiol antioxidants in SCD deserves further investigation.
Rijneveld: Jazz pharmaceuticals: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Delannoy: Innate Pharma: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal